Abstract
Introduction
Ponatinib at a daily dose of 45 mg is an orally available TKI approved for patients failing or not tolerating first- and second-line therapy in CML-CP. Although ponatinib showed robust response rates in CML-CP patients highly resistant and intolerant to second-generation TKIs it was not recommended for earlier lines of therapy in the past primarily due to cardiovascular toxicities. The OPTIC trial demonstrated durable efficacy and improved safety with a response-based dose adjustment strategy, starting with 45 mg and reducing to 15 mg upon achievement of ≤1% BCR-ABL1 (Cortes et al. Blood 2021;138:2042-50).
Methods
In the present PONS study (NCT03807479), we exclusively investigated ponatinib starting with 30 mg/d in second-line and mostly after second-generation TKI. To prospectively capture treatment related cardiovascular events, patients were monitored with echocardiography, duplex or ankle brachial index of lower extremities, blood sugar metabolism and fundoscopy at baseline and 12 weeks. The primary endpoint was rate of MMR by 12 months of ponatinib. Dose escalation in patients not responding up to 45 mg was allowed.
Results
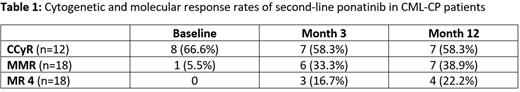
A total of 22 patients were screened and 18 (14 male and 4 female) were enrolled from December 2018 until January 2022, including ten resistant and eight intolerant patients to first-line TKI treatment. At baseline, the median age was 43.6 years (range, 20 to 79). Prior to therapy with ponatinib, only one (5.5%) patient was in MMR, eight (66.6%) were in CCyR (complete cytogenetic remission) and two (16.65%) were in MCyR (major cytogenetic remission). First-line treatments were imatinib (n=3), dasatinib (n=9), or nilotinib (n=6).
The median duration of ponatinib treatment was 14.5 months (2.4-26.3). In 17 patients, the starting dose of ponatinib was 30 mg/d.
For interim analysis, response and toxicity data of months 3 and 12 (n=18 patients) were analyzed. One patient showed data consistent with lymphatic blast crisis at baseline but was kept on study. After 3 months, 7 (58.3%) patients with available cytogenetics analyses were in CCyR, 6 (33.3%) in MMR and 3 (16.7%) in MR4 or better. After 12 months, 7 (58.3%) patients were in CCyR, 7 (38.9%) in MMR and 4 (22.2%) in MR4 or better. At the time of this analysis (data cut 01. Jun 2022), all patients were alive and one patient progressed to advanced CML.
Currently, 6 patients are still under treatment, 4 are past end of study treatment, 4 discontinued treatment due to adverse events (AEs), 1 failed to achieve a CHR at months 3, 2 were switched to an alternative treatment, and 1 because of protocol deviation.
Toxicity data at month 3 showed a total of 86 AEs (all grades) with 15 considered as grade 3/4. Of these, 15 AEs 3 were hematological and 12 were non-hematological. Grade I/II cardiovascular AEs were hypertension (n=6), coronary artery disease (n=1), and stent implantation (n=1). Only 1 grade 3 cardiovascular event (aphasia) occurred.
Data on toxicity prevention including echocardiography, duplex or ankle brachial index of lower extremities, blood sugar metabolism, and fundoscopy will be presented.
Conclusion
In patients failing first-line second-generation TKI therapy with nilotinib or dasatinib, we observed robust responses and fewer cardiovascular events in patients treated with a reduced dose of ponatinib. Importantly, strict preventative measures of cardiovascular risk factors may reduce the occurrence of such AEs, providing durable disease control.
Disclosures
le Coutre:Pfizer: Honoraria; Novartis: Honoraria; Incyte: Honoraria. Göthert:Proteros Biostructures: Honoraria; zr pharma&: Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Ariad: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; AOP Pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Travel support; Abbvie: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Imago Bioscience: Membership on an entity's Board of Directors or advisory committees. Saussele:Incyte: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Roche: Honoraria; Pfizer: Honoraria. Bullinger:Astellas: Honoraria; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene/BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bayer Oncology: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Elmaagacli:Novartis: Honoraria; BMS: Honoraria; Incyte: Honoraria. Stegelmann:Incyte: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; AOP Pharma: Consultancy, Honoraria. Hochhaus:Bristol Myers Squibb: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Incyte: Research Funding. Ernst:Janssen: Other: travel grant. Burchert:Incyte: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AOP Health: Honoraria, Research Funding.
OffLabel Disclosure:
Despite significant progress in the treatment of patients with chronic phase CML, there is still need to further optimize therapy to reach the goal of disease eradication for almost all patients. In case of imatinib failure, dasatinib and nilotinib are effective treatment options after an individualized treatment selection. Although MMR rates of around 30% after 2 years of therapy are a significant achievement, options that may improve response rates in depth are still desirable. Ponatinib is a third generation TKI with very high anti-clonal activity in all CML phases. Moreover, it also eradicates most of the known and problematic mutations and only very few (compound) mutations may induce ponatinib-resistance. Based on its favorable target spectrum, it is expected that Ponatinib may be more effective than 2nd line dasatinib or nilotinib in achieving early (i.e., at 6 months) cytogenetic and molecular responses in patients after inappropriate response to imatinib, and more effective as 2nd line treatment after failure of initial treatment with dasatinib or nilotinib than a cross-over between the 2nd generation TKIs. The basic hypothesis underlying therapeutic programs in CML is to be able to achieve meaningful and long-lasting suppression of the Philadelphia chromosome and breakpoint cluster region-abelson fusion gen (BCR-ABL). Complete cytogenetic responses have been associated with improved survival in CML, while major molecular responses are associated with improved event-free survival.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal